The only medical cannabis product in the Australian Register of Therapeutic Goods might soon be joined by another.
Australian health regulators quietly gave Epidiolex, a cannabidiol medication, orphan designation and priority review status in late 2019.
The designations allow the Therapeutic Goods Administration to waive application and evaluation fees, as well as expedite its assessment of the application to list Epidiolex in the ARTG, the Department of Health disclosed in a recent public submission to a Senate committee.
Inclusion in the ARTG would represent a milestone – and not only because only one other cannabinoid medication is listed.
An ARTG listing also makes a medicine eligible to apply to be added to the Pharmaceutical Benefits Scheme – the mechanism for government subsidy of prescription medicines.
The only medical cannabis product currently listed in the ARTG is Sativex, which was approved in 2012.
Both Sativex and Epidiolex are made by United Kingdom-based GW Pharmaceuticals.
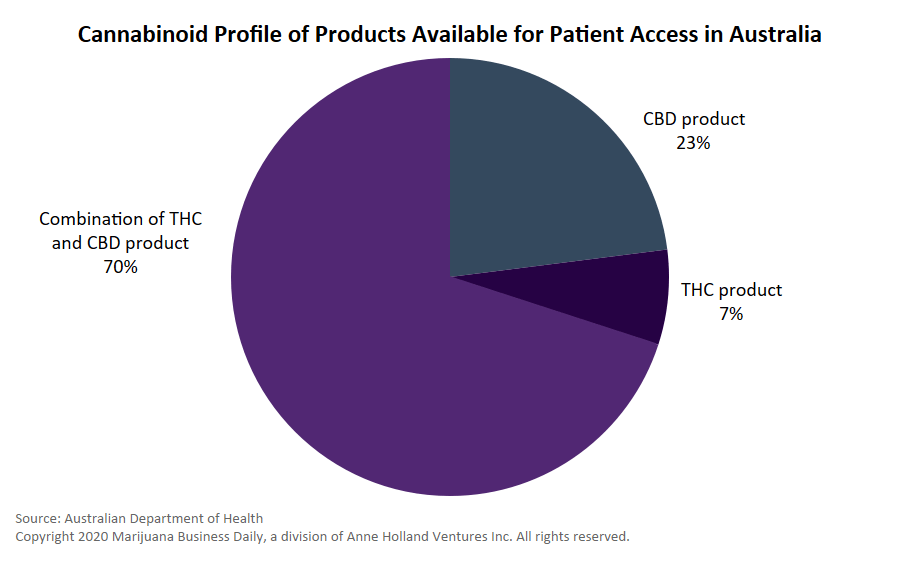
All other medical cannabis products remain in the “unapproved” category.
Through the end of 2019, roughly 130-plus medical cannabis products were prescribed by 1,400 medical practitioners to treat over 130 conditions.
However, Australia aims to approve more medical cannabis for specific treatments.
“While most prescriptions so far have been written for unapproved products, the government’s ultimate goal is to have a wider range of medicinal cannabis products included in the Australian Register of Therapeutic Goods as registered medicines,” according to the disclosure.
Before a medicine can be registered, the Therapeutic Goods Administration reviews its quality, safety and efficacy for a certain condition.
“Although there are a number of clinical trials of medicinal cannabis products for different conditions underway in Australia and internationally, at present there are still only a limited number of well-designed published studies assessing the efficacy of medicinal cannabis products for different (conditions),” the submission notes.
“Neither priority review determination nor orphan drug designation guarantee registration on the ARTG,” according to the filing.
The health department did not immediately reply to a query from Marijuana Business Daily.
GW Pharmaceuticals trades on the Nasdaq (GWPH) and the London Stock Exchange.
Matt Lamers is Marijuana Business Daily’s international editor, based near Toronto. He can be reached at mattl@mjbizdaily.com.