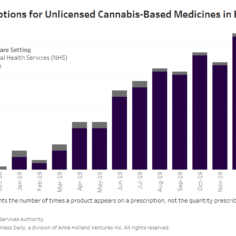
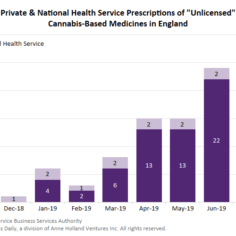
England’s medical cannabis market shows little growth as of early 2020
England’s medical cannabis market has barely gotten off the ground since launching about 19 months ago, with a two-tier system in which few patients access products through legal channels while most resort to illicit suppliers.