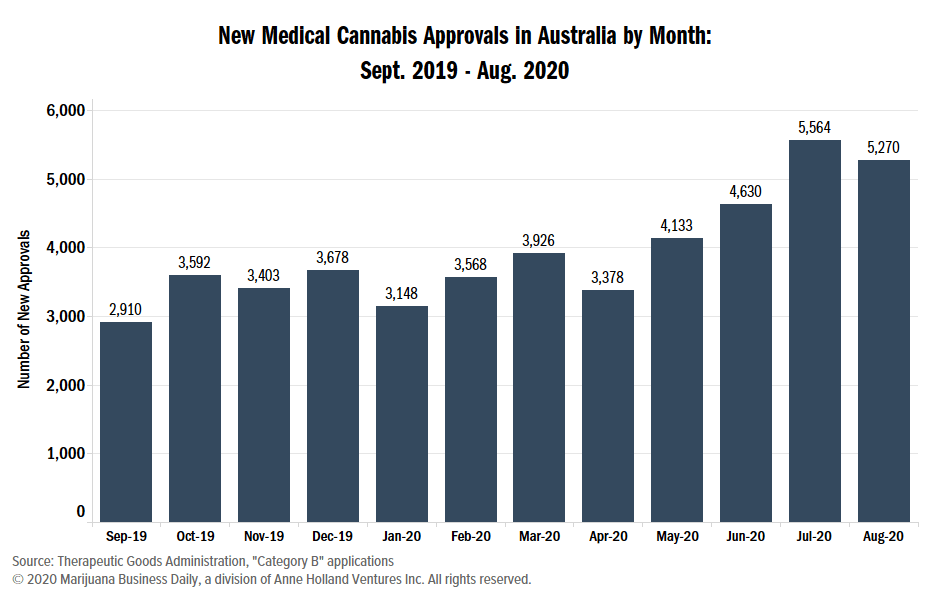
Roughly 5,270 patients were approved for medical cannabis treatment in Australia in August via the SAS Category B pathway, the second month in a row the country recorded more than 5,000 individual approvals.
The August figure represents a 5% drop from the previous month.
Most medical marijuana prescriptions in Australia are under the SAS B pathway.
In the meantime, regulators at the Ministry of Health moved closer to allowing over-the-counter sales of cannabidiol products.
The Therapeutic Goods Administration (TGA) announced an interim decision this week to amend the country’s Poisons Standard and down-schedule CBD to a Schedule 3 product in oral, oral mucosal and sublingual formulas for therapeutic use.
CBD is currently a Schedule 4 substance, making it available only with a prescription.
CBD products could be sold without a prescription if they adhere to certain criteria, per the interim decision.
The interim decision includes a new appendix entry for CBD that would restrict the supply of CBD to products listed in the Australian Register of Therapeutic Goods (ARTG) to ensure that only those approved for Schedule 3 will be available without a prescription.
“The majority of the members on the committee were not persuaded that there is currently sufficient evidence to relax the access controls on cannabidiol, derived from plants or synthetically produced, to the general sales levels,” the announcement said.
The proposed implementation date for the down-scheduling is June 1, 2021.
Comments on the interim decision can be submitted by Oct. 13.
A final decision on down-scheduling has been set for Nov. 25.
The Australian patient data can be found here.
Hemp Industry Daily contributed to this report.