Australia has proposed amendments to the Narcotic Drugs Act that would overhaul the medical cannabis industry by streamlining the licensing process for businesses and ensure access for patients.
Rhys Cohen, editor-at-large for the Australian publication Cannabiz, said the measures will allow companies to operate more efficiently and flexibly if fully implemented.
“For foreign businesses, it reduces the cost and risk of setting up Australian-based licensed operations. And globally, once these changes are implemented and refined, it means Australian exports will be even more competitive,” he told Marijuana Business Daily.
The Narcotic Drugs Amendment (Medicinal Cannabis) Bill 2021 was introduced last week in the House of Representatives, where it awaits a first vote.
The proposed amendments would implement some of the key recommendations outlined in the McMillan Review of the country’s medical cannabis industry in 2019.
In particular, the bill seeks to:
- Consolidate the licensing structure into a single license, replacing the current three permits.
- Implement a “perpetual” license framework where validity periods will no longer be specified.
- Reaffirm the Australian government’s commitment to patient access of medical cannabis products.
That would “reduce the regulatory burden for industry participants undertaking activities across the spectrum of regulated activities – cultivation, production, manufacture and research,” according to an explanatory memorandum of the proposed changes.
Another main part of the bill, according to the memorandum, would shift supply-chain assessments to later in the application process, rather than earlier in the licensing stage, “to support the long term nature of business investment decisions.”
Cohen said the level of information currently required by the government at the initial application stage has caused bottlenecks and therefore challenges for businesses.
“These are amendments to the Narcotic Drugs Act, but it’s not fiddling around the edges: These are substantial reforms to the framework,” he said.
“These reforms have been in the works for some time and underwent extensive industry consultation. I expect this bill will pass quickly with bipartisan support.”
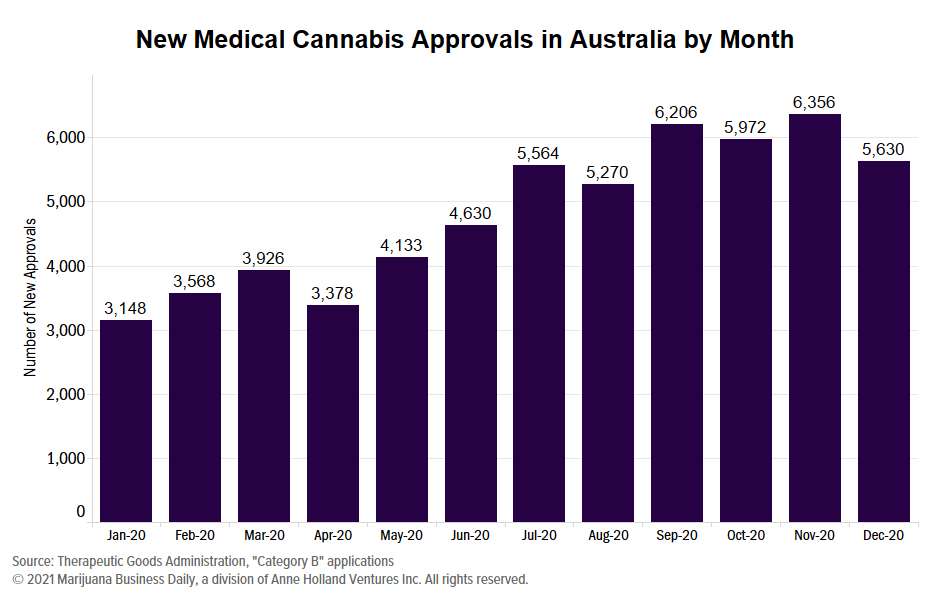
Australia ended the fourth quarter of 2020 with 17,958 medical cannabis approvals, up from the previous quarter’s 17,040 approvals.
The proposed bill is available here.