Executives welcomed recommendations to improve the framework governing Australia’s medical cannabis scheme as a potential breakthrough, but some said the expected implementation of the proposals is “far too slow” for a sector in need of regulatory improvement today.
The recommendations – made in a government-commissioned report authored by John McMillan, law professor at Australian National University in Canberra – aim to address the regulatory burden facing businesses, which has been a hurdle in the early years of the medicinal cannabis scheme.
One way the government can achieve that is aggregating three current licenses into one, the report suggests. Faster turnaround times and more industry engagement are also part of the proposals.
The report acknowledges that regulators received a “substantially larger number” of business license applications than anticipated and the Office of Drug Control (ODC) was not adequately resourced.
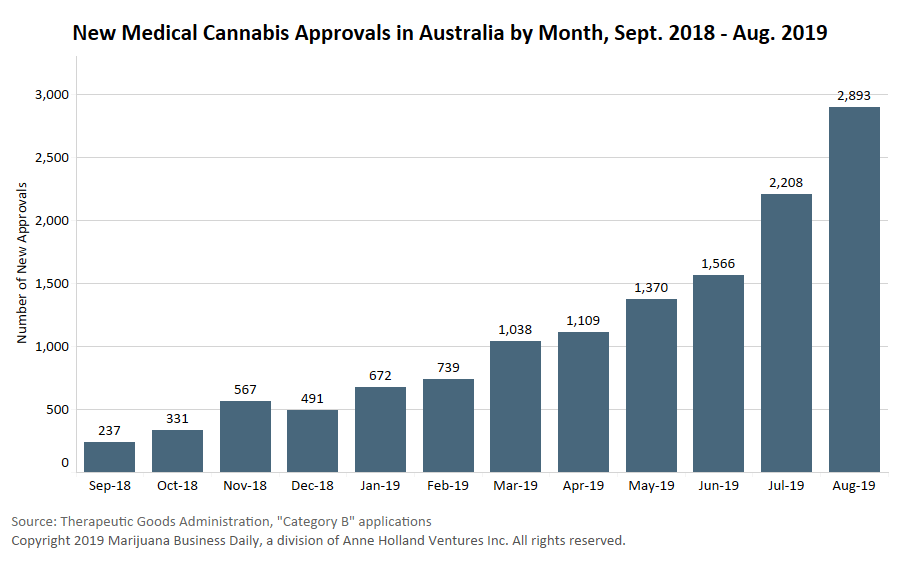
As of this summer, 246 license applications were submitted, with only 24 medical cannabis licenses granted, plus 16 for research and 23 for manufacture.
“This (total applications) is a far higher number than expected. It points to strong commercial interest in the Australian medicinal cannabis industry,” McMillan wrote in his report, which says the scheme “has overall been resoundingly successful.”
Last year, Health Minister Greg Hunt appointed McMillan to review the 2016 amendments to 1967’s Narcotic Drugs Act, which laid the legal footing for the country’s medical cannabis industry.
The final report was released earlier this month with 26 recommendations.
According to the Office of Drug Control (ODC), some of the reforms will be implemented “later this year,” but that leaves most of the 26 recommendations for 2020 or later.
“It’s wonderful these recommendations have been published; however let’s remember, these are only recommendations and it will all boil down to the details,” said Adam Miller, managing director of the consultancy BuddingTech.
“These changes are the start of much-needed change, and the sooner it happens the better.”
Australia saw a record 2,893 patient approvals in August. (See chart.) However, McMillan’s report acknowledged that the country’s scheme remains “in its early days.”
Key recommendations
Some of the proposals could solve long-running problems plaguing businesses.
For instance, recommendation No. 11 looks to reduce the amount of detail and specificity required for permits.
“Permitting operations is inconsistent due to the complexities that regulators are required to oversee, so simplification will hasten the process and assist all concerned,” Russell Harding, founder and CEO of MedReleaf Australia, said in an interview with Marijuana Business Daily.
McMillan also recommends aggregating three current licenses – cultivation, manufacture and research – into one.
That makes “eminent sense,” said Harding, “as much of the criteria overlap, including a reduction in the number and breadth of mandatory requirements.”
Miller said the current separate license system creates layers of paperwork for applicants and the ODC.
“As a result, the information overload has led to the ODC taking close to two years to process applications,” he said. “By creating a unified license structure, this will decrease the lead time for approvals due to less paperwork.
“Faster turnaround times lead to several positive outcomes for industry, for example, the ability for companies to raise capital – due to application approval (key performance indicators) – and more importantly, the ability to get plants into the ground faster, and therefore building a local supply for patients.”
Another proposal would extend the term of a medicinal cannabis license, research licence or manufacture license to a maximum of five years.
The report also proposes a review of the ODC’s administrative procedures to provide better service to existing license holders.
McMillan identified submissions from stakeholders to that end, including:
- The appointment of ODC case managers or liaison officers to licensees.
- Allowing minor license and permit variations to be approved upon notification.
- Having a fast-track procedure for manufacture licence applicants who already hold a medical cannabis license.
- Holding regular industry consultation forums.
CBD
The report’s second recommendation deals with cannabidiol, requesting that CBD be removed from the Narcotic Drugs Regulation 2016.
That would mean manufacturers could use pure CBD as an ingredient without an ODC license.
“This would be consistent with other Australian practice,” the report stated.
For example, the Therapeutic Goods Administration reclassified CBD in July 2015 from being a prohibited substance in Schedule 9 to being a prescription medicine in Schedule 4.
Rhys Cohen, director of Cannabis Consulting Australia, said the CBD change would make it easier to manufacture CBD products, but it won’t change how they are accessed or prescribed.
A GMP certification would still be required.
“This provides a vehicle for manufactures in Australia to enter the CBD market and could quite possibly reduce the cost of CBD products for patients across Australia,” said Miller.
Timing
In his report, McMillan wrote that the improvements should be implemented “as early as practicable.”
When reached via email by Marijuana Business Daily, McMillan declined to answer questions on any preferred timing for his report’s implementation, saying only he will “leave it to government to decide whether and how to implement my recommendations.”
Industry sources are already concerned implementation of some of the recommendations could stretch into 2021.
“It is far too slow,” said Harding. “Many of the recommendations do not require parliamentary engagement, so should be implemented as soon as possible.”
The ODC proposed a vague two-stage implementation process, with “some” of the recommendations being implemented this year.
“Some reforms can be made immediately, others will take more time, and some will require legislative change,” said Cohen of Cannabis Consulting Australia.
“For example, the unification of licenses will require a change to the Act. But the Review is very explicit that the government shouldn’t wait to enact whatever improvements they can immediately.”
Cohen would like to see a road map from the ODC explaining when certain reforms will be made.
“Saying some reforms will be made this year could mean a single minor reform is made late December,” he said.
Understanding when government will make certain regulatory changes will help businesses plan for the future.
Not addressed
The report stopped short of offering solutions for longstanding issues with the medical cannabis scheme.
Fleta Solomon, managing director of Little Green Pharma, said the industry faces tight advertising and promotion restrictions by the TGA due to medical cannabis being an “unregistered” product and having to be accessed through the Special Access Scheme.
“Medicinal cannabis is not, and will unlikely to be anytime soon, available on the Pharmaceutical Benefits Scheme,” she said.
Affordability for patients, prescribing complexity and doctor education remain unresolved issues.
“We’re still in baby step land, contrary to the hype,” Harding said.