(This story has been updated to correct that Validcare provided a study to the FDA.)
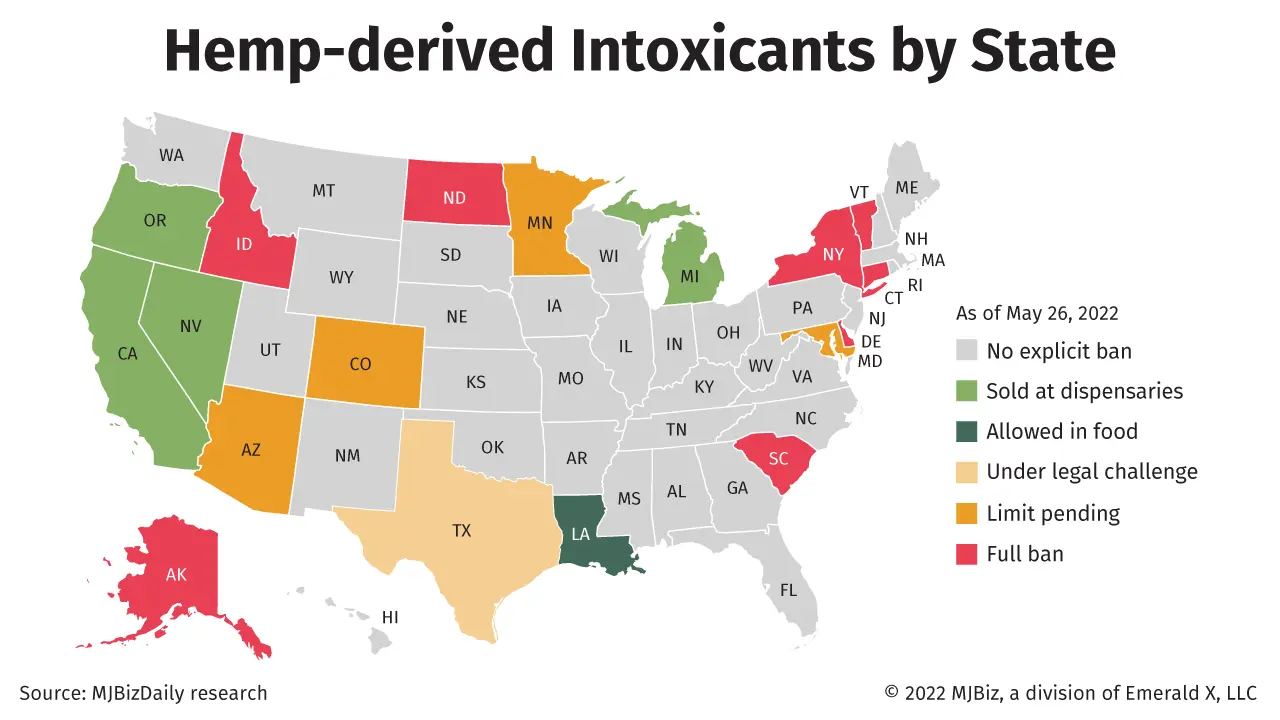
Cannabinoid manufacturers have less than a week to submit data and comments to U.S. health regulators considering whether to allow extracts such as CBD into foods and dietary supplements.
The Science Board of the U.S. Food and Drug Administration, an advisory panel made up of nutrition and drug-safety experts, is meeting June 14 to discuss “challenges in evaluating the safety of dietary supplement and food ingredients with predicted pharmacological activity, utilizing cannabinoids as a case study.”
The scientists are expected to focus largely on hemp-derived CBD.
Public comments and other studies will be accepted for consideration through June 7.
The FDA has held open a public docket on the safety of CBD since 2019, when the agency held its first public meeting to consider over-the-counter cannabinoid products.
Since then, the agency has repeatedly said there isn’t enough research to show that CBD and other cannabis extracts are safe.
Are you a social equity cannabis license holder or applicant?
The MJBizCon team is now accepting 2023 Social Equity Scholarship Program applications.
The mission of this program is to provide social equity cannabis license holders or applicants access to the #1 global cannabis industry conference + tradeshow in Las Vegas.
Who can apply?
- Students currently enrolled in a cannabis-related program at an accredited university or college.
- Cannabis executives at licensed social equity cultivation, extraction/processing, retail, manufacturing/brand businesses (or awaiting application approval).
Don’t miss out on this potentially life-changing opportunity.
Apply to attend MJBizCon today – The application period will close on July 24!
The June 14 Science Board CBD review is the first since Denver-based Validcare sent the FDA the results of an observational study refuting some of the FDA’s stated concerns about CBD, including the possibility of liver toxicity and lowered testosterone in males.
Marc Scheineson, an attorney and former FDA official, told Pharma Intelligence that if the scientists find the Hemp Roundtable data to be sufficient, they could consider allowing over-the-counter CBD in lower doses than the levels found in prescription CBD drugs.