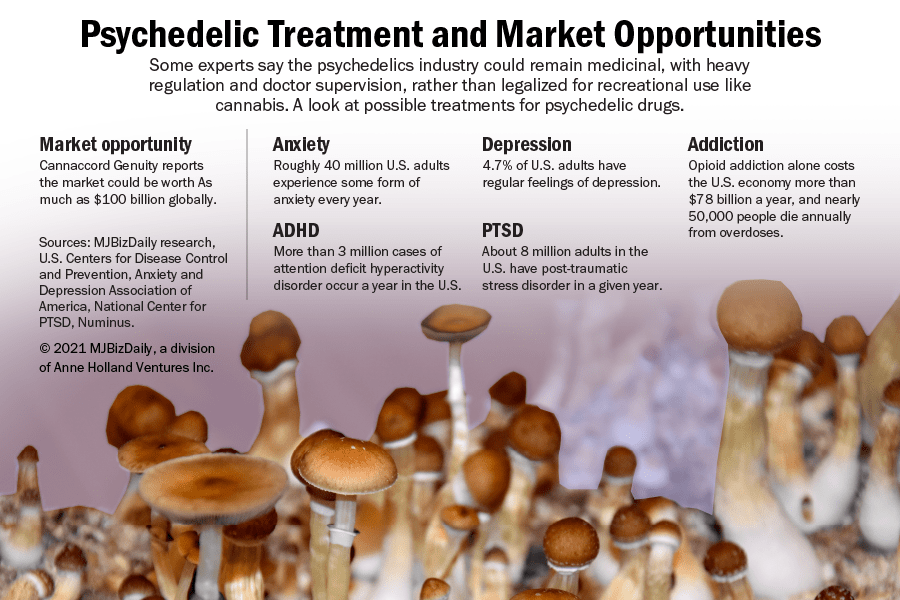
The nascent psychedelics industry is taking a different path to market than the marijuana sector, despite their shared medicinal roots.
While most states moved to regulate commercial medical marijuana programs without an extensive library of research, psychedelics advocates are adopting a different strategy.
“In the U.S., cannabis went for legalization in states before (there was) a ton of research,” explained Dr. Lynn Marie Morski, a former physician with the U.S. Department of Veterans Affairs who now serves as president of the Psychedelic Medicine Association.
“It led with the legalization, whereas psychedelics led with the research,” added Morski, who will speak about emerging research on the medical applications of psychedelics at The Emerald Conference, April 1-3 in San Diego.
Follow the research
Though scientists began studying pharmacological compounds known as psychedelics as early as the 1950s, it took more than 40 years to find their place in academia.
In 2000, researchers from John Hopkins University in Baltimore were the first to obtain regulatory approval to reinitiate research with psychedelics.
The researchers’ 2006 landmark study highlighting the safety and long-term benefits of a single dose of psilocybin – the psychoactive ingredient in magic mushrooms – ushered in a new era of research.
In 2019, the Johns Hopkins School of Medicine established the Center for Psychedelic & Consciousness Research (CPCR), the first institute of its kind in the nation.
To date, CPCR and university researchers have published more than 150 peer-reviewed articles in scientific journals.
Several other schools, including Stanford University, have become research leaders in the expanding field.
“There was so much research on psychedelics before even one state, Oregon, had it even partially legal,” Morski told MJBizDaily.
In November 2020, Oregon voters approved Measure 109 to create a program for administering psilocybin products to those 21 and older.
In September 2023, the Oregon Health Authority granted a license to Epic Healing, making it the first regulated psilocybin service provider in the state.
Charting a new path
After nine years as a physician at the VA, Morski left to focus on educating other health care professionals such as clinicians, doctors, nurses and therapists about the benefits of psychedelic medicines.

Her role as a defense contractor and psilocybin’s Schedule 1 status as a federally prohibited substance precluded Morski from even raising the topic of psychedelic therapy treatments with VA patients – despite growing research demonstrating the value of psychedelics when addressing the root cause of trauma, reducing symptoms of post-traumatic stress disorder (PTSD) and improving traumatic brain-injury symptoms.
“I couldn’t go to work and tell the veterans who needed this the most,” Morski said.
Inspired to create change, Morski helped establish the Psychedelic Medicine Association in San Diego to bridge the gap between traditional medical care and psychedelic therapies through data, education and research.
“We formed to educate clinicians on psychedelics so that they feel comfortable having those conversations with patients,” Morski said.
Business opportunities
The private sector is playing a key role in advancing psychedelics science, leveraging years of academic research to create new studies and commercialize various drug treatments that incorporate ketamine, MDMA and psilocybin.
Lykos Therapeutics, formerly known as MAPS Public Benefit Corp., is among the leaders in the field.
The San Jose, California-based company has completed two Phase 3 trials for PTSD and submitted a new drug application to the U.S. Food and Drug Administration for MDMA (midomafetamine capsules) to be used in combination with psychological intervention, including talk therapy and other supportive services from qualified health care providers.
The FDA is expected to conclude its review in August.
MDMA-assisted therapy has not been approved by any U.S. regulatory agency.
If the FDA were to approve it, the U.S. Drug Enforcement Administration would be required to reschedule MDMA for medical use. MDMA has been classified as Schedule 1 since 1985.
In January, Lykos announced it raised more than $100 million in a Series A funding round led by Helena, a Los Angeles-based global investor.
Betty Aldworth, communications director for the California-based Multidisciplinary Association for Psychedelic Studies, highlights the key role that private industry will play in the emerging sector.
“While drug developers, ancillary service providers, training providers, retreat centers, university research centers, clinic networks, insurers and many other larger-scale businesses will drive the eye-popping industry size estimates for psychedelics,” she said, “psychedelic-assisted therapies and treatments are fundamentally a person- and community-oriented practice.
“Clinicians facilitate the treatments, local nonprofit clinics are structuring their businesses for equitable access and researchers are exploring the cutting edge of psychedelic-assisted treatments in small proof-of-concept studies to establish new areas for research.”
MDMA clinics will be established, according to Morski, similar to the advent of ketamine clinics in the late 2000s.
Insurers will play a key role for businesses as advocates lobby for coverage for these expensive therapies, she added.
“There will definitely be a boom of psychedelic-related business, for sure,” Morski said.
Chris Casacchia can be reached at chris.casacchia@mjbizdaily.com.