The regulatory body for therapeutic goods in Australia is reviewing complaints involving the noncompliant advertising of medical cannabis products.
The Therapeutic Goods Administration (TGA) says it “has written to a number of entities in relation to noncompliance with the law with instruction to cease unlawful behaviour.”
Criminal offences and civil penalties may apply to breaches of the Narcotic Drugs Act and the Therapeutic Goods Act, the TGA said.
The review is prioritizing complaints where consumers “may be at risk because the medicinal cannabis products offered for sale have not been through regulatory checks,” the TGA said.
Rhys Cohen, principal consultant for FreshLeaf Analytics, wrote that the advertising of regulated medicines is strictly controlled in Australia,
“The TGA clarified how these regulations apply to the medical cannabis industry back in November 2019. In short, it is a breach of the regulations to advertise or promote the use of a specific prescription medicine or a class of prescription medicines to the general public,” according to Rhys.
The investigation into unlawful cannabis advertising comes as the TGA expands the number of quality inspections for imported medical cannabis.
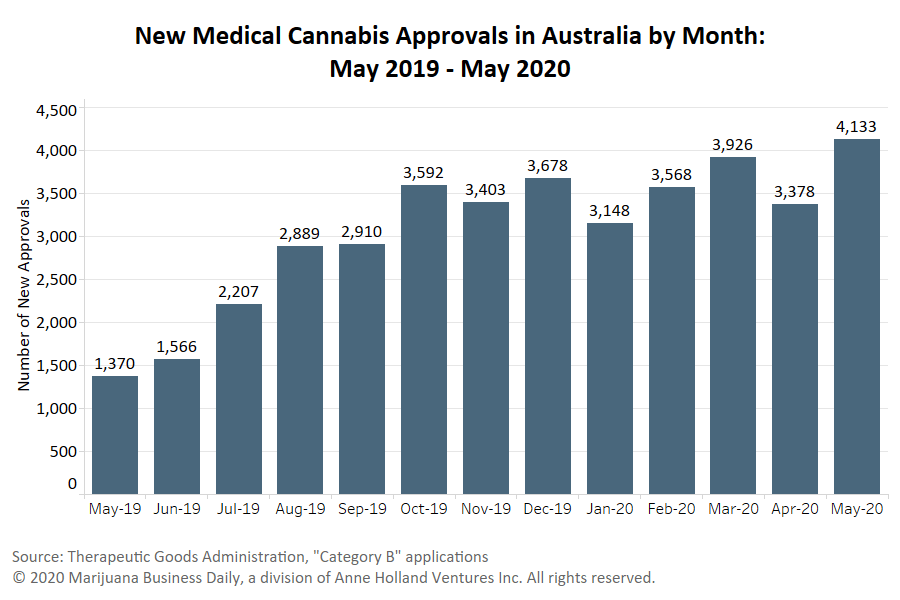
Most Australian medical cannabis is still imported as local producers continue to ramp up production.
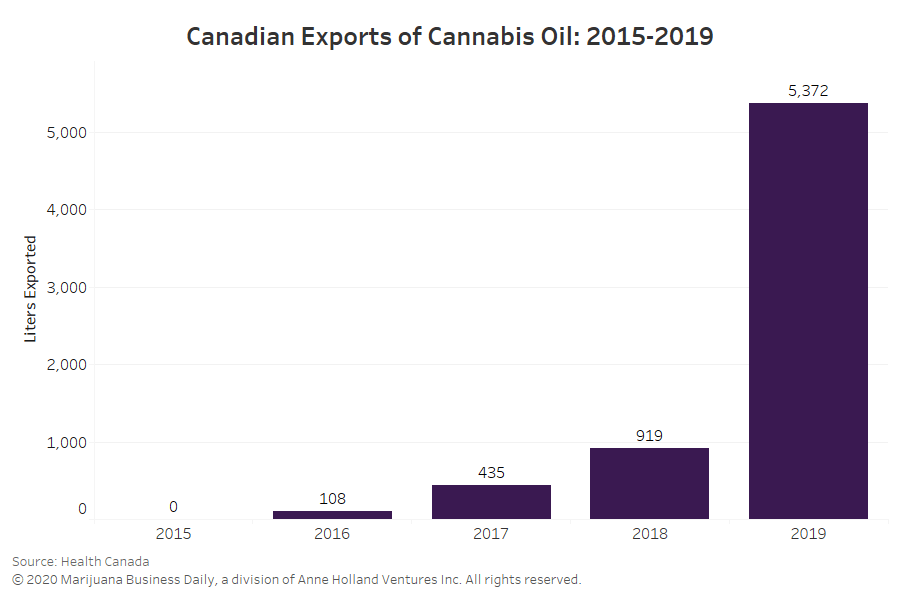
In 2019, Australia imported some 3,700 liters (977 gallons) of medical cannabis oil products as well as 204 kilograms of flower from Canada for medical and scientific use.